 Enlarge
Enlarge
British Mineralogy
Native Lime
- Class 2. Earth*.
- Order. 1. Homogeneous. Bab.
- Gen. 1. Lime.
- Spec. 1. Calx nativa.
- Gen. Char. Powdery or concrete, with a hot burning taste. Corrodes animal substances. Spec. grav. 2.3, Kirwan, v. 1. 5. Precipitates from a solution in water, by adding corrosive sublimate, in the form of a reddish powder. Kir. v. 1. 75. Changes syrup of violets green.
- Spec. Char. Uncombined.
- Syn.
- Native lime. Kir. v. 1. 74, 75.
- Pure lime. Bab. 7.
- Artificial. Calx viva. Mat. Med.
Quick-lime, or Calx viva, is well known, as procured from chalk or lime-stone by means of burning in lime-kilns. In the act of burning it is deprived of an air or gas, chemically termed carbonic acid gas†, loses part of its weight, and takes up caloric, or latent heat of Dr. Black. It is then caustic, with the properties as described in the generic character, changing the syrup of violets green. This character it retains as long as the latent heat or the effect of it lasts, which heat and principle of changing the syrup of violets green will be lost if exposed to a damp atmosphere.
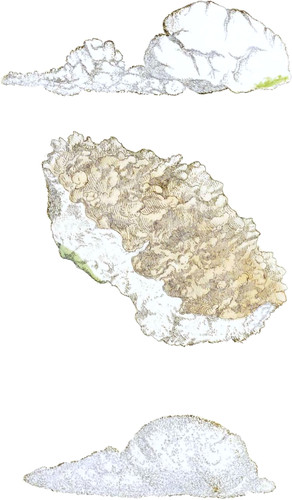
The upper figure is done to express artificial lime just exposed to damp air, yet capable of changing the syrup of violets green, and beginning to fall to pieces. If a quantity is suddenly added, it will lose its characteristic property sooner, by absorbing carbonic acid gas from the atmosphere, or the water of which the fire had deprived it in the kiln, and when dried without heat will be nearly what it was at first.
The middle figure, Calx nativa, from Bath, has qualities resembling quick-lime, and changes syrup of violets to a green, nearly as vivid as that produced by the artificial lime above; and although I have had it two years in the drawer with quick-lime, it still gives a green which the other does not.
The lower figure represents lime taken out of a hollow nodules of flint, to which, before it was broken, we could find no apparent aperture. The contents were exposed immediately to some fresh violet petals, pressed so as to afford two or three drops of purple fluid, which it directly changed green. It soon lost that property, and is not a gritty chalk.
External Character of the Bath Lime.
- Colour white.
- Lustre 0.
- Transparency 0.
- Fracture earthy.
- Hardness, rubs easily to powder.
It should seem that this passes out of the rocks in a fermentative manner, oozing or frothing. The upper surface of the specimen is somewhat encrusted with a stalactitical substance. The inner part when examined seems partly in bubbles.
Dr. Moreton found lime in the stones of Cliftone pit in Northumberland‡, and Dr. Falconer at Bath. Sir John Hill describes a similar substance to mine, which he has seen thrown out of the quarries of Mr. Allen near Bath, and calls it native lime and Gypsum Tymphaciumof the antients, saying that Theophrastus has left a record of a ship taking fire from the heating of the gypsum among some clothes that were in it, on the accidental admission of wet; and that he does not call it gypsum hiself, but an earth only that the people about Tymphæa, &c. called gypsum.
- * Earths are incombustible, infusable per se, spec. grav. not exceeding 4.9, and white.
- † Formerly termed fixed air, discovered by Dr. Black. It is heavier than common air, forming a small or adventitious part of the atmosphere; is readily absorbed by cold water, giving it a brisk taste. As an acid, it turns vegetable blues red.
- ‡ Since the above was written, Mr. John Hailstone, Woodwardian Professor, of Cambridge, kindly informs me that the Calx nativa sent to Dr. Woodward by Dr. Moreton has no pretensions to be a lime.

