 Enlarge
Enlarge
British Mineralogy
Carbonate of Barytes
- Class 2. Earths.
- Order 1. Homogeneous.
- Gen. 6. Barytes.
- Spec. 1. Carbonate of Barytes.
- Div. 1. Crystallized.
- Gen. Char. Pulverulent, white, somewhat pungent. Spec. Grav. 400. Soluble in most of the acids, and in 900 times its weight of water. Its nitrate does not tinge flame red. its sulphate is nearly soluble. It forms a hepar with sulphur, which is poisonous. Bab.
- Spec. Char. Combined with carbonic acid.
- Syn.
- Barolite or aërated baryites. Kirw. v. 1. 134.
- Witherite. Syst. Min. Jameson, p. 573.
- Witherit. Emmerl. v. 1. 546. Werner.
- Baryte carbonatée. Haüy, v. 2. 308.
We received the fine specimen here figured, from the leadmine of F. Hall, Esq. at Arkendale, near Richmond, Yorkshire, by favour of our friend the Rev. J. Harriman, in December 1803. We have since received specimens, from the same place, from Mr. W. Watson of Bakewell, which he gathered in September 1803. It was first found at Anglesark in Lancashire only, but has since been observed at several other places.
Carbonate of barytes, it appears, was first discovered by Dr. Withering (see Phil. Trans. for 1784, 301.), when it was called aërated barytes; but Mr. Werner, wishing to honour Dr. Withering for his abilities and accuracy, named it Witherite. It has since very properly been called carbonate of barytes. Radiating cabonate of barytes, in its weight and appearance, very much resembles carbonate of strontia: however, it differs from it in never being of a greenish colour, and in having its radii larger, more compact, and flatter.
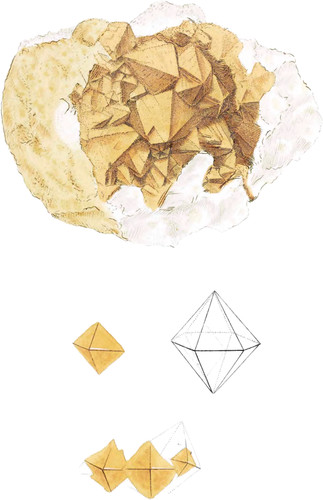
The upper figure represents carbonate of barytes in dodecaëdral crystals, formed of two hexaëdral pyramids joined base to base, like quartz.
These are the largest I have seen, and are very rare at present. They are covered with a light ochraceous substance, perhaps calamine. The matrix is carbonate of barytes, in part decomposed, and of a chalky appearance. The figures below show the geometrical plan, and in what manner one of the solid angles o the base has been mistaken for part of an octaëdron, or has given the idea of two four-sided pyramids joined base to base, which many have described as one of its forms of crystallization.

