 Enlarge
Enlarge
British Mineralogy
Carbonate of Lime
- Div. 1. Crystallized.
The perhaps numberless variations in the forms of the crystals of Carbonate of Lime, however difficult they may at first be considered by many, are one of the surest proofs of the necessity of attending particularly to their formation; for when we are a little conversant with them, they seldom fail to indicate the place to which a specimen should be referred.
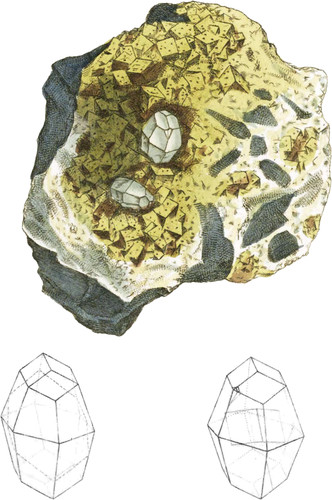
The present variety is formed of two regular hexaëdral pyramids applied base to base, each terminated by the faces of the equiaxe rhomb—see tab. 34—the alternate solid angles near the summit, being bevelled, form the little that remains of the metastatic variety—see tab. 33. The incidence of the three edges of the termination on the corresponding edges of the pyramid is about 122°, that of the face of termination on the other three is nearly 133°, and the two pyramids on each other about 145°. The crystals are apt to vary a little, especially those in the next plate, for which these measures will serve.
The right hand geometrical figure in this plate shows the position of the primitive rhomb in the centre, and the left hand one shows the oblique base of the curious mackle that belongs to this modification, to assist in the explanation of the next plate.
The crystals are extremely neat on this specimen, which is in the possession of Mr. Lowry, whose remarkable abilities are so well known. It came from Ecton-mine, in Staffordshire. The same gentleman has a group of crystals of the same form from nearly the same spot; one crystal about ten inches in circumference, and nearly as long.
The present specimen is rendered handsome, and serves two purposes here, by giving an example of yellow fluor. There are also some varieties of octaëdral Pyrites, a few little spots of Galæna, &c. The whole stand on a piece of shattered schistose Limestone.

