 Enlarge
Enlarge
Exotic Mineralogy
Magnesian Lime
- Syn.
- Magnesian Lime. Brit. Min. Syst. Index. tab. 217. 402, 497.
- Compound spar. Muricalcite. Kirw. I. 92.
- Chaux Carbanatée Magnésifère. Haüy II. 187. Tabl. 5.
- Dolomite. Kirw. I. 111.
- Chaux carbonatée aluminifère. Haüy II. 173.
- Rautenspath and Dolomite of Werner.
- Picrite. Brong. I. 230.
- Carbonate of Lime and Magnesia. Aikin, 164.
- Bitterspar. Nonnul.
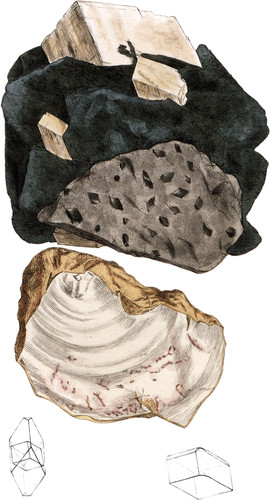
The crystallized varieties of this mineral have been long known to contain Magnesia, and from the bitter taste of the salt, consequently produced by dissolving it in Sulphuric acid, it has Obtained the name of Bitter spar. Since the presence of Magnesia gives a form of nucleus different from that of Carbonate of Lime, although very nearly allied to it, it has been admitted by modern mineralogists as a well defined speeies, and is found to be liable to many of the various forms that are assumed by other minerals, in different states of aggregation, and most of these forms have received peculiar names; thus it occurs crystallized in several modifications, the Rautenspath of Werner; lenticular, and of a greenish colour, Miemite; massive, with a granular structure, Dolomite and Magnesian Limestone, and compact, with a slight transparency, when it is called Gurhofite, or Gurhofian or Conite, &c. some of these varieties being named from the places where they were first found, often before their component parts were known. The Dolomite, Magnesian Limestone, and Flexible Limestone of Sunderland, all varieties of this species, have been figured in British Mineralogy; the present plate exhibits the primitive crystal, a rhomb whose angles measure 106°15 and 73°45, imbedded in green foliated Talc, or Chlorite; the acute rhomb, produced by a modification upon the lateral solid angles, receding upon the faces of the nucleus, towards the apex, and sometimes the apex is truncated: these crystals are imbedded in a granular mass of the same substance; it is from Halle in Saxony; and a variegated piece of a compact variety from Meissner in Hossia, called Conit, or Dichter Bitterkalk by the Germans, for which I am indebted to my valuable correspondent the Professor Stromeyer.
The constituent parts vary very much in different varieties, some containing little else than Carbonate of Lime, and Carbonate of Magnesia; others holding much Carbonate of Iron; the latter approach in the form and lustre, as well as composition, to pearly Brown-spar (Carbonate of Iron in curving rhombs) as the Carbonate of Magnesia is diminished in quantity the Iron is increased, and Manganese often introduced; thus rendering it difficult to determine which species many such intermediate specimens belong to; in general, however, those belonging to the Brown-spar (Carbonate of Iron) become black, when subjected to the action of the blow-pipe, and produce a dark green glass when fused with Borax: they are also less readily soluble in muriatic acid than even Magnesian Lime, which itself does not dissolve so quickly as Carbonate of Lime, as is manifested by the slower evolution of gas.

