 Enlarge
Enlarge
British Mineralogy
Carbonate of Lime
- Div. 1. Crystallized.
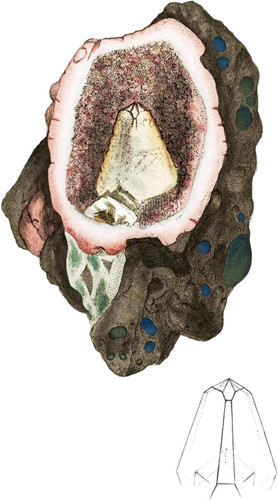
The hills of Pentland, near Edinburgh , are famous for Petunse*, and much variety in Mineralogy; also for Agates and other Pebbles. I am obliged to Mr. Jackson, who was botanizing in that neighbourhood, for this specimen; for, although he docs not study mineralogy, he was struck by the. singular appearance of this stone when he picked it up, and the regular formation of the Carbonate of Lime placed so distinctly within the hollow is certainly worthy of notice; for we know of no theory which satisfactorily accounts for such a formation. The surrounding pinky Quartz, in bundles of little eighteen-sided crystals, lines the cavity, and the jasperine Quartz (if I may so call the red coat) seems to terminate the whole pebble as it were, which is surrounded by part of the rock of a brown hue, called trap, in which there are smaller or larger pebbles sometimes included, and sometimes hollows where others have been entrapped. These hollows are sometimes coated with a green or blue earthy substance called by some the Green Earth of Verau, probably owing to an uncertain mixture of Iron: this occasionally coats also the stones included. The Carbonate of Lime is composed of half of a very acute rhomb with three largish faces of the æquiaxe, and three smaller ones, probably belonging to the primitive rhomb: see geometrical figure.
It is not a little singular that the like stones excluding the trap have been found in Wiltshire at a small depth under ground, of which I have obtained a specimen by favour of the Marchioness of Bath.
This specimen, rich with information, is particularly worth the attention as well of the novice as of the adept, considering the curious divisions of formation in the different substances of which it is composed. The part of the rock this came from seems to have been a mixture, as it were a chaotic one, (if I may so term it,) appearing like the fragments of various rocks that had undergone the action of moist elements, so as to form air, and water bubbles, which could not immediately escape. Apparently a continual deposition has taken place, more still forming, and enclosing the preceding till the vvhoVe matter was deposited. In the mean time each elementary substance, according to the particular formation of its molecules, and the nature of its nearest neighbour, formed, either by itself or into combination. Thus the Carbonic Acid and Lime united together, so as to construct a Crystal in the middle of this hollow as complete as circumstances would admit of, depending on the quantity of Carbonate of Lime received in solution, perfecting some faces and depositing the other molecules irregularly, A small tinge of Iron stained the solvent, and consequently the Crystal towards the top is a little coloured. The surrounding Quartz has also crystallized under similar circumstances, and is somewhat stained with the Oxide of Iron among the Crystals, giving this lining a pinkish hue, which is again conspicuous at the outer side and edge next the piece of compound rock.
- * An interesting substance used in porcelain.

