 Enlarge
Enlarge
British Mineralogy
Sulphate of Lime
- Class 2. Earths.
- Ord. 1. Homogeneous.
- Gen. 3. Lime.
- Spec. 4. Sulphate of Lime.
- Sect. 2. Common Sulphate of Lime.
- Div. 3. Amorphous.
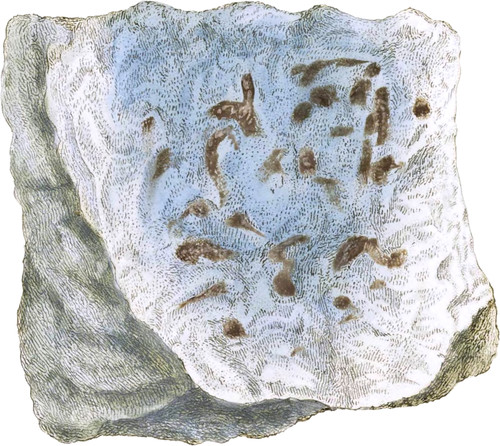
The lower figures of Tab. 236, exhibit the common red and white varieties of Amorphous Gypsum. That part of the present specimen which is of a blue colour has by some been considered as a variety of the Anhydrous Sulphate of Lime; (see Exotic Mineralogy, tab. 39,) so ably described by the Count de Bournon in the Transactions of the Geological Society, under the name Bardiglione*. Its softness and the considerable loss in weight which it suffers upon being heated red hot, determine it to belong to the common Sulphate of Lime; or, as the Count de Bournon would express it, the Hydro-sulphate of Lime.
Blue is a colour extremely scarce in Gypsum, and it is tolerably bright in this specimen; it does not appear to have been noticed by any author. The outside of the mass of which the figure represents a part, is very soft and nearly opaque; the inside is harder and more transparent as it approaches the blue, with which it is gradually blended; the blue in the centre being the hardest part. I obtained this specimen at a warehouse in which immense quantities of Gypsum had been collected from Nottinghamshire to be manufactured into manure.
It is sometimes found greenish, &c. as at Bilton, near Knaresboroughj by my friend Dr. P. Murray.
- * In this paper the Count hints at the probable existence of Bardiglione in the Salt mimes of Cheshire. I should be glad to find this suggestion verified.

