 Enlarge
Enlarge
Exotic Mineralogy
Anhydrous Sulphate of Lime
- Syn.
- Chaux sulfatée anhydre. Haüy 4. 348.
- Anhydrite, Würfelspath. Werner.
- Bardiglione, or Sulphate of Lime. Bournon in Trans. of Geological Society, 1. 355.
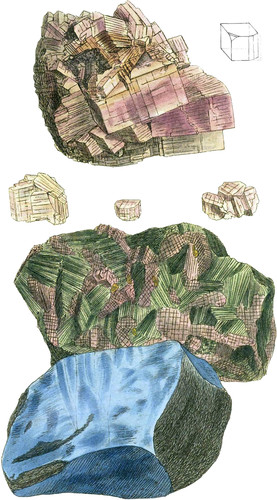
I am glad to have an opportunity of showing figures of this very interesting substance, which at present is rare, probably because it is not yet universally enough known, although, after the able investigation by the very experienced Count de Bournon, in the Geological Society’s Transactions, it may be found to be more generally distributed, as he expects. It appears to the Count, to use his own words, “not to be confined to the secondary strata, but also to occur in some veins in primitive rocks, such as that variety from Pesai, near Mont Blanc, as well as that from Sweden, Hitherto it has been met with much more frequently in the newer rock-formations, particularly those that include large deposits of Sea-salt, as at Bex, Hall, Weiliezka, Arbonne, Carinthia, Upper Austria, Swabia,” &c. The upper figure is from a good specimen of the crystallized variety, from a salt-mine in Saxony, favoured by Dr. Babington; it contains, besides imperfect rectangular prisms, many of which are fractured, one crystal, part of which only is exposed to view, whose solid angle is replaced by an isoceles triangular plane; the angles, however, from its situation, could not be precisely measured; nevertheless, it proves what the Count had suggested, that the nucleus is not a cube. This is the only specimen, at present known, that exhibits a modification upon the solid angle. Near this crystal is beautiful minute mackle of common Gypsum, the same as figured Brit. Min. tab. 233. and in various parts are small portions of greyish clay. The smaller crystals are from the Counts own collection; theyexhibit two or three of the 15 varieties of the six modifications upon the vertical edges figured by him. I have exhibited below the variety from Sweden, mixed with Actinolite and Copper Pyrites: it is the specimen mentioned by the Count as formerly in Mr. Greville’s collection. I have added a specimen of the blue variety: both these are from the British Museum.
This substance is somewhat harder than Carbonate of Lime; is easily broken into rectangular fragments, with flat polished surfaces; the lustre is very considerable, and sometimes slightly pearly. Some specimens were found by the Count to be phosphorescent; some electric. Its refractive powers are peculiar; a line drawn upon one side of a clear piece, when viewed perpendicularly through the opposite side, appears single, but if viewed obliquely it is doubled; hence probably the reason for the difference between Bournon and Haüy. The nucleus is divisible in the direction of both the diagonals of its base: Bournon observes, that the lines within the crystals, indicating this division, appear to intersect each other at an angle of nearly 100°; whereas fractured surfaces, apparently parallel to these striæ, measured upon the primitive faces, give 135°; so that they would intersect each other at 90°. This circumstance he is inclined to attribute to the refracting power of the substance; and hence concludes, that a triëdral prism, with a rectangular isoceles triangle for its base, is the true integrant molecule. The Spec. Grav. when crystallized, according to Haüy, 2.964, or to Bournon, 2957. It is not acted upon by acids, and is difficultly fusible into a white brittle enamel. It occurs in lamellar, granular, compact, and stalactitiform masses. The lamellar variety is often sufficiently compact to be used by statuaries, as is the case at Bergamo and Milan. It differs chemically from common Sulphate of Lime, in not containing any water, of which the common Sulphate contains 22 per cent.
| Analysis by Vauquelin. | By Klaproth. | ||
|---|---|---|---|
| Lime | 40 | 41.75 | |
| Sulphuric Acid | 60 | 55. | |
| Muriate of Soda | 1. | ||
| Loss | 2.25 | ||
| 100 | 100.00 | ||

