 Enlarge
Enlarge
Exotic Mineralogy
Boracite, Borate of Magnesia
- Syn.
- Magnésie boratée. Haüy 2. 337. Tabl. comp. 16.
- Borazit. Emmerl. 1. 509.
- Quartz cubique. Journ. de. Physique, Oct. 1788, p. 301.
- Calx combined with Boric Acid. Kirw. 1. 172.
- La Boracite. Brochant 1. 589.
- Boracite. Sowerby’s Catalogue, pt. 1. p. 25.
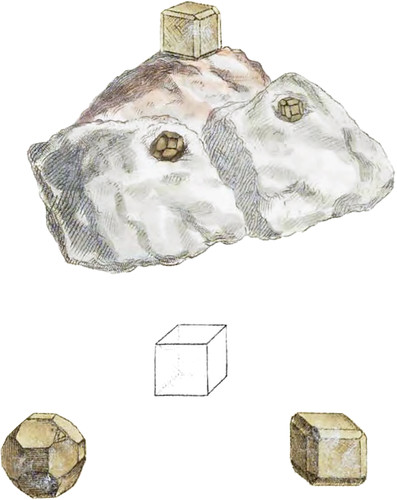
This has been a long time known, from being found on the Hill of Kalkberg, near Lunaberg, in Hanover, and nowhere else. It is found in a bed or mass of Gypsum, and, I believe, it has not been found for some years, it is therefore chiefly in old collections. I am obliged to some former Hanoverian friends for my specimens. Lazeus, who first discovered it, I presume from the form and hardness, called it Cubic Quartz, for it is harder than might be expected from the contents upon analysis. It forms crystals, either perfect cubes, or cubes with the eight solid angles truncated, passing to the cubo-octaëdron, and often nearly to the octaëdron; the edges are also generally truncated, nearly so deep as to produce the rhomboidal dodecaëdron, but neither the octaëdron or dodecaëdron form perfectly; the truncations upon the angles are often alternately large and small, &c. I herewith figure those I have of the natural size, geometrically arranged as above, for a memorandum, and as an instructive lesson on crystallization. They are seldom much larger: colour greyish; lustre dull vitreous., It will scratch Fluate of Lime; it is brittle, mostly full of flaws. Spec. Grav. according to Westrumb 2.556.
| Boracic Acid | 68.00 |
| Magnesia | 13.50 |
| Lime | 11.00 |
| Silica | 2.00 |
| Alumina | 1.00 |
| Oxide of Iron | 0.75 |
| 96.25 |
The Lime has been shown by Vauquelin and Schmit to be combined with Carbonic acid, and variable in quantity, so it may be considered as accidental, as the crystals examined were not transparent.
When heated it becomes electric, froths before the blow-pipe, gives a greenish light, and becomes a yellowish enamel, illiniting in small points, which by a continued heat are united in bright sparks.
I annex a geometrical outline of a larger size than the crystals themselves, which includes all the facets or faces known. Fig. 1. shows the face r of Haüy.

