 Enlarge
Enlarge
Exotic Mineralogy
Antimonial Sulphuret of Silver, or Red Silver
- Syn.
- Argent antimonié sulfuré. Haüy, Tabl. 75. Traité 3. 402.
- Mine d’argent rouge. De Lisle, 4. 447.
- Roth Gultigerz. Emmerl. 2. 185.
- Red Silver ore. Kirwan, 2. 122.
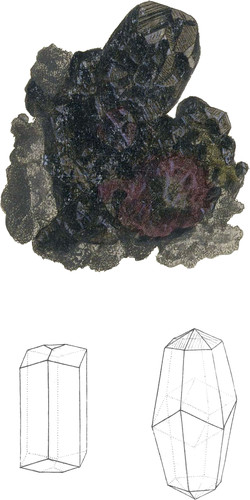
This species of Silver Ore has been long known by the name of Red Silver Ore; it is found in the mines of Germany, Hungary, &c. The fine specimen, from which this figure is taken, enriches the Cabinet of my obliging friend W. E. Rundell, Esq. and is one of the best known, some of the crystals being very large, and others nicely defined; they rest upon irregularly crystallized Galæna, mixed with a little Carbonate of Lime.
The primitive crystal of this substance is, according to Hauy, an obtuse rhomb, the angles of whose faces are 104° 28′ and 75° 32′, and their inclination upon each other 109° 28′ and 70° 32′. The colour is various shades of dull red, so intense in the present specimen, as to appear nearly black. This substance is semi-transparent, soft, rather brittle, easily powdered; powder red. The Spec. Gray, is from 3.563 to 3.608. Under the action of the blow-pipe it melts before it is red-hot, anti when red, it gives out fœtid vapours without flame*, and shortly becomes reduced to a while metallic globule of Silver.
The crystals are commonly hexaëdral prisms, or double hexaëdral pyramids, terminated by three or six-sided summits.—See the outlines.
Klaproth and Vauquelin have considered the metal, of which it is composed, as in combination both with Oxygen and Sulphur; and Proust, on the other liand, denies the presence of Oxygen in Red Silver; this latter opinion appears to be confirmed by synthesis. I have ascertained, that if a mixture of Silver, Antimony, and Sulphur be heated to redness in a glass retort, they immediately combine†, forming a vitreous, very fusible, dark red, and semi-transparent mass, very much resembling the darker varieties; if Silver, Arsenic and Sulphur be used, a similar combination is obtained, differing, however, in being more brittle, more transparent, of a lighter colour, and possessing more lustre: this compound burns with a blue flame, when heated red-hot, which the other does not, unless it contains some uncombined Sulphur. Prout’s analysis, and what has just been observed, seem sufficient to support Werner in making two species, although, according to Haüy, there is but one; and the Arsenic mentioned by some Authors is considered by him as probably derived from the matrix.
| Sulphuret of Silver | 58 |
| ————— Antimony | 33 |
| Oxide of Iron | 3 |
| Sand | 3 |
| Loss | 3 |
| 100 |
| Sulphuret of Silver | 74.35 |
| ————— Arsenic | 25.00 |
| Oxide of Iron | 0.65 |
| 100.00 |
- * Werner makes two sub-species of Red Silver ore, the light and the dark; the specimen here figured, I presume, belongs to the latter, as it does not inflame before the blow-pipe; I also conceive it to contain Antimony, not Arsenic.
- † If a deficiency of Sulphur is used, the excess of metals forms a separate button; if an excess of Sulphur be used, it is distilled over.
- ‡ Should I be so fortunate as to meet with an handsome specimen of this species, 1 shall be glad to exhibit a figure of it.

