 Enlarge
Enlarge
British Mineralogy
Oxide of Zinc, or Calamine
- Class 3. Metals.
- Order 1. Homogeneous.
- Gen. 6. Zincum.
- Spec. 1. Oxygenizatum.
- Div. 2. Imitative.
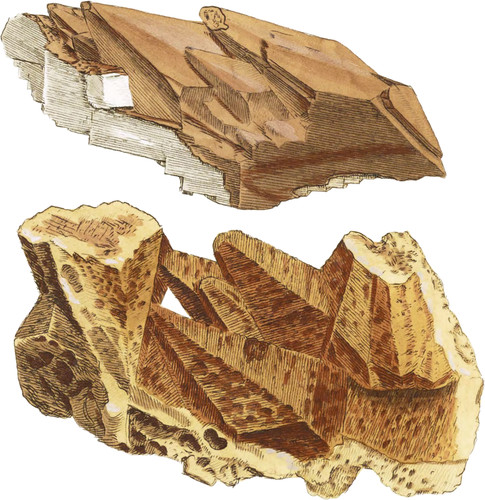
Oxide of Zinc, tab. 156, was crystallized in a shape peculiar to itself. In the present instance it occurs in the form of another substance, viz. Carbonate of Lime: see tab. 34. It is not a little remarkable that this oxide should thus take the place of another substance, and assume its form, so as to become what are termed secondary crystals*. They are so frequent in Oxide of Zinc as often to prove a very convenient help towards discriminating that substance, otherwise not easily characterized, from the earthy appearance it commonly assumes. It is found in Flintshire†, Derbyshire, and at Mendip in Somersetshire, as well as in several other parts of the United Kingdoms, in these shapes, mostly taking the form of Carbonate of Lime, and is often detected in the process shown in the upper figure. The upper surface is a smoothish Oxide of Zinc, and beneath still remains crystallized Carbonate of Lime. In the lower figure the Oxide of Zinc has supplanted the Carbonate of lime, and is cellular or porous, which is one of its characters, whence it is often called bony, from its resemblance to the cellular inner part of a bone. It is sometimes white, but mostly coloured by Oxide of Iron, with various ochrey tints, and seldom has any lustre. It is procured in large quantities for the manufacture of brass, &c., and produces about thirty per cent of Zinc‡.
- * When any mineral takes the place of a crystal, either by decomposing it or taking the cast of the mould first formed by another, it is called secondary, as it is so to those formed originally by the first substance. Calamine sometimes replaces Cubic fluor, &c.
- † Whence I have received good specimens from D. Pennant, Esq., son of the well-known Pennant, author of British Zoology, and the Rev. H. Davies
of Beaumaris. - ‡ Zinc has been found perfectly ductile if heated to a certain temperature.

